Call: or Contact Us
- Home
- About Us
-
Products
- Pre-treatment Chemicals
-
Electroplating Intermediates
- Acid Copper Plating Intermediates
- Acid Zinc Plating Intermediates
- Alkaline Zinc Plating Intermediates
- Bright Nickel Plating Intermediates
- Silver Plating Intermediates
- Nickel Plating Intermediates
- Zinc Plating Intermediates
- Copper Plating Intermediates
- Tin Plating Intermediates
- Chrome Plating Intermediates
- Alloy Plating Intermediates
- Zincate Zinc Plating Intermediates
- Potassium Chloride Zinc Plating Intermediates
- Hardware Acid Copper Plating Intermediates
- Circuit Board Acid Copper Plating Intermediates
- Acid Tin Plating Intermediates
- Other Plating Intermediates
- Electroplating Additives
- Post-treatment Chemicals
-
Others
-
Electroplating Chemicals
- Alloy Plating Chemicals
- Cobalt Plating Chemicals
- Complexing Agent for PCB Plating
- Copper Plating Chemicals
- Methane Disulfonic Acid And Its Salts
- Methane Trisulfonic Acid And Its Salts
- Nickel Plating Chemicals
- Nickel Salt for Plating
- Selenium Plating Chemicals
- Sulfo Acetic Acid & Its Salts
- Tin Plating Chemicals
- Tin salt for Plating
- Zinc Plating Chemicals
- Other Plating Chemicals
- Electro-plating Solution
- Plating Process
-
Electroplating Chemicals
- Services
- Recommendation
- Base Materials
- Applications
- Resource
- Contact Us

![{[CurrentData.Name]}](https://resource.alfa-chemistry.com/structure/6046-93-1.gif)
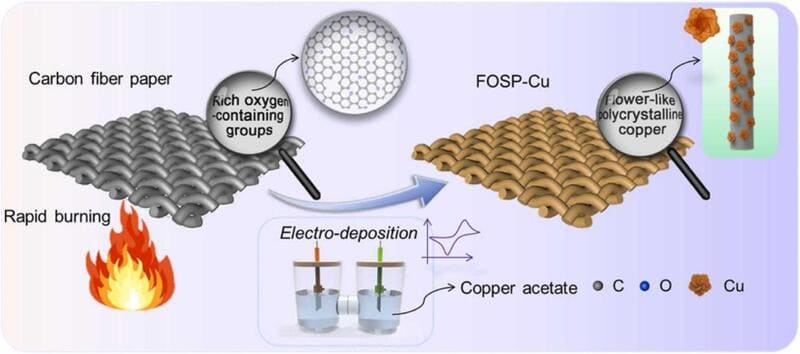 Zhao, Yaling, et al. Nano Energy 97 (2022): 107124.
Zhao, Yaling, et al. Nano Energy 97 (2022): 107124.
 Jabeen, Saadia, et al. Applied Nanoscience 11 (2021): 79-90.
Jabeen, Saadia, et al. Applied Nanoscience 11 (2021): 79-90.
 Kumar, J. Sharath, et al. New Journal of Chemistry 42.5 (2018): 3574-3581.
Kumar, J. Sharath, et al. New Journal of Chemistry 42.5 (2018): 3574-3581.
